Two buffer solutions, `A` and `B`, each made acetic acid and sodium acetate differ in their `pH`... - YouTube

SOLVED: Acetic acid (CH3COOH, 𝐾a=1.80×10−5) is a weak acid, so the salt sodium acetate (CH3COONa) acts as a weak base. Calculate the pH of a 0.809 M solution of sodium acetate. pH=

Sodium Acetate(CH3COONa) - Structure, Properties, Preparations, Uses, Important questions, FAQs of sodium acetate.
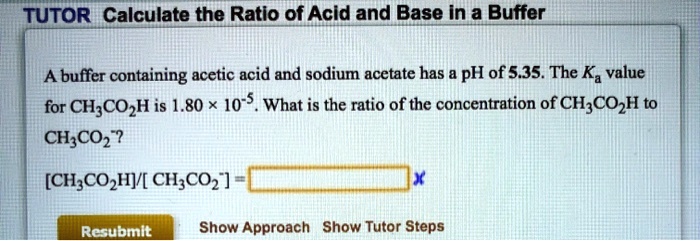
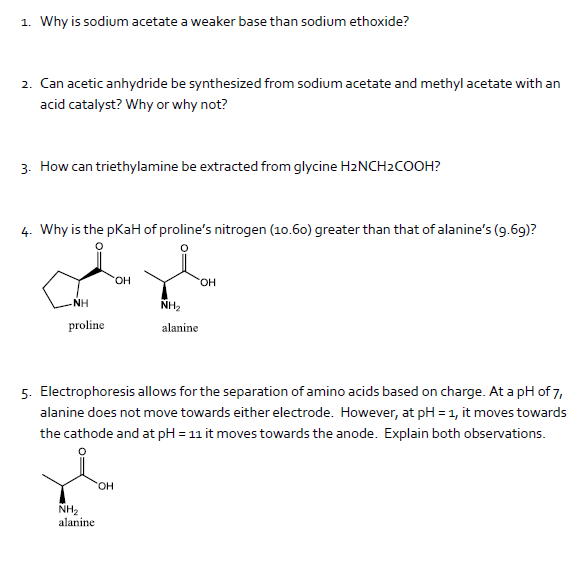
SOLVED: TUTOR Calculate the Ratio of Acid and Base In a Buffer A buffer containing acetic acid and sodium acetate has pH of 5.35. The Ka value for CH;COzH is 1.80 x

1 Chapter 10 Acids and Bases 10.9 Buffers. 2 When an acid or base is added to water, the pH changes drastically. A buffer solution resists a change in. - ppt download

SCH 4 U 1. What are buffers? Buffers are mixtures of conjugate acid- base pairs that allow a solution to resist changes in pH when acids and/or bases. - ppt download

If sodium acetate is a weak acid and does not readily dissociate in water or completely and a strong electrolyte is defined as the oppposite how come the answer is B and

SOLVED: Sodium acetate (NaC2H3O2) is a basic salt. When sodium acetate is dissolved in water, it dissociates into its component ions. This reaction goes to completion, as indicated by the one-way arrow

In a mixture of acetic acid and sodium acetate, the ratio of concentrations of the salt to the acid is increased ten times. Then the pH of the solution:

You have 250mL of a 0.56M solution of sodium acetate. How many mL of 0.50M acetic acid should be added to make a buffer of pH 4.40? | Homework.Study.com
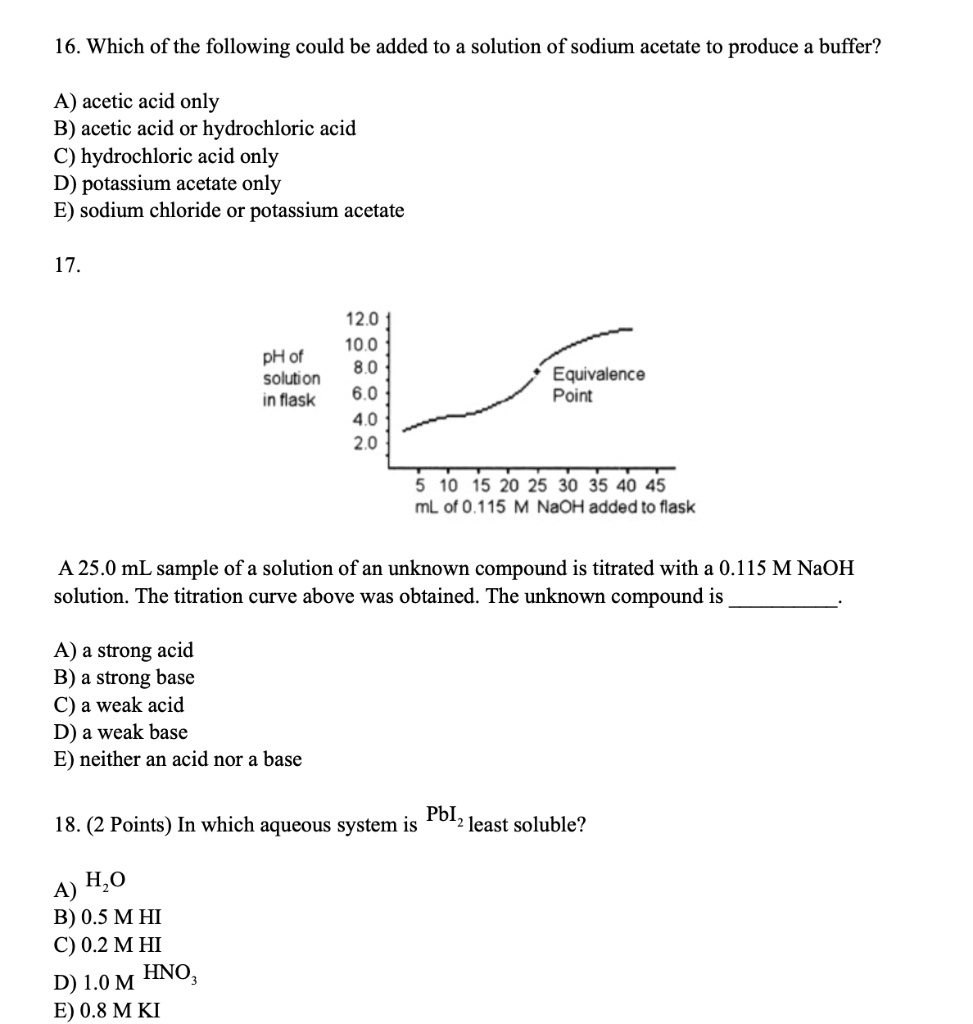
SOLVED: 16. Which of the following could be added to a solution of sodium acetate to produce a buffer? A) acetic acid only B) acetic acid or hydrochloric acid C) hydrochloric acid
Why does the solution of sodium acetate give more concentration of Hydroxide ion? Shouldn't the number of Hydroxide ion and hydrogen ion be equal? - Quora